Applied Cell Biology
In the research focus of the Applied Cell Biology, we investigate space-induced changes in the cellular mechanisms of neuronal cells. We use model systems relevant to human health, such as human and mouse cells, personalised stem cells and organoids derived from them. To gain further insights into the underlying mechanisms and to identify countermeasures against microgravity-related deficits, we use both real microgravity and ground-based simulation facilities that replicate space conditions such as microgravity, hypergravity, ionising radiation, and altered atmospheric conditions.
Countermeasures for neural changes in space and on Earth
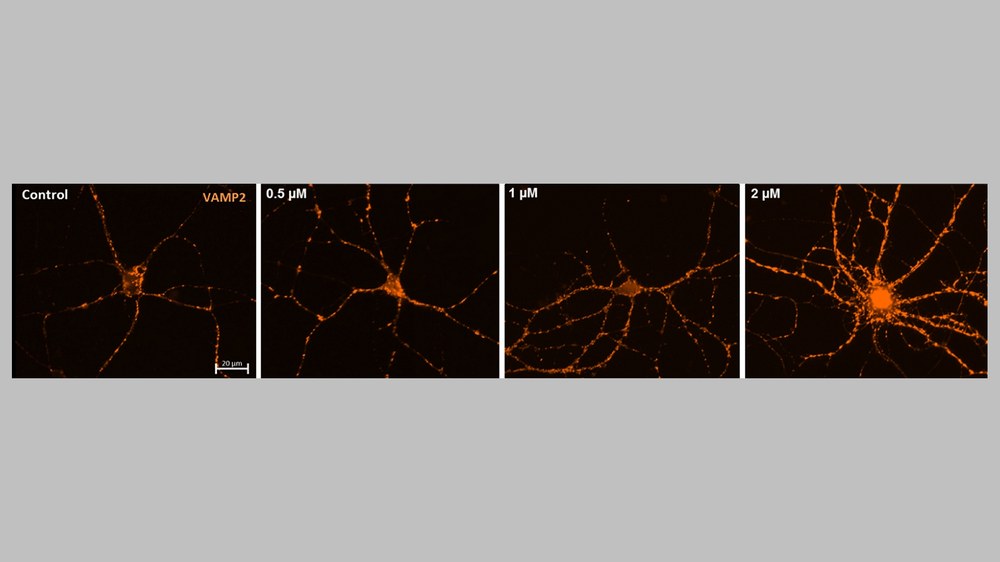
Extreme environmental conditions, such as those found in space, can impair essential neural structures and functions of the human brain. Therefore, the development of appropriate neuroprotective agents for future manned space missions is of crucial importance. The loss of synaptic plasticity in the brain, for example, is directly linked to cognitive deficits and therefore offers a promising target for therapeutic intervention. The neurostimulant drug Ketamine is already used to treat treatment-resistant depression, but in addition to its stimulating effect on synaptic growth, it also has significant psychotropic side effects. Recently, its metabolite, Hydroxynorketamine (HNK), has been attributed with a similar effect on synaptic plasticity, whose weak affinity for NMDA glutamate receptors also led to a reduction in psychotropic side effects.
The aim of research in this field, in collaboration with the Technical University of Cologne, is to utilise and optimise the effect of Ketamine and Ketamine-derivatives on synaptic plasticity for astronauts as well as for patients on Earth.
Preliminary results from microscopic examinations and electrophysiological measurements, show that potential disturbances in synaptic plasticity could possibly be mitigated by the new compounds. In addition to their use in space travel, the novel derivatives also have the potential to be developed into an improved therapy for neurodegenerative diseases on Earth.
FLUMIAS: High-resolution 3D fluorescence microscope for live cell imaging on the ISS
FLUMIAS is a live cell fluorescence microscope to investigate changes in metabolic processes, membrane dynamics, and behaviour in living cells and microorganisms in real time on the ISS. With FLUMIAS, we aim to better understand the effects of altered gravity conditions at the microscopic level. By using fluorescent dyes and markers, structures within human and animal cells can be visualised in order to examine living cells over longer periods of time and to visualise them as 4D models. The FLUMIAS microscope will be installed on a centrifuge on the ISS in 2026, allowing samples to be observed in microgravity, but also under up to 1g, i.e. Earth's acceleration. In addition to the microscope unit, FLUMIAS includes a life support system that keeps the samples at the right incubation temperature and supplies them with nutrients via a pump system.
The LAARA experiment on board the ISS
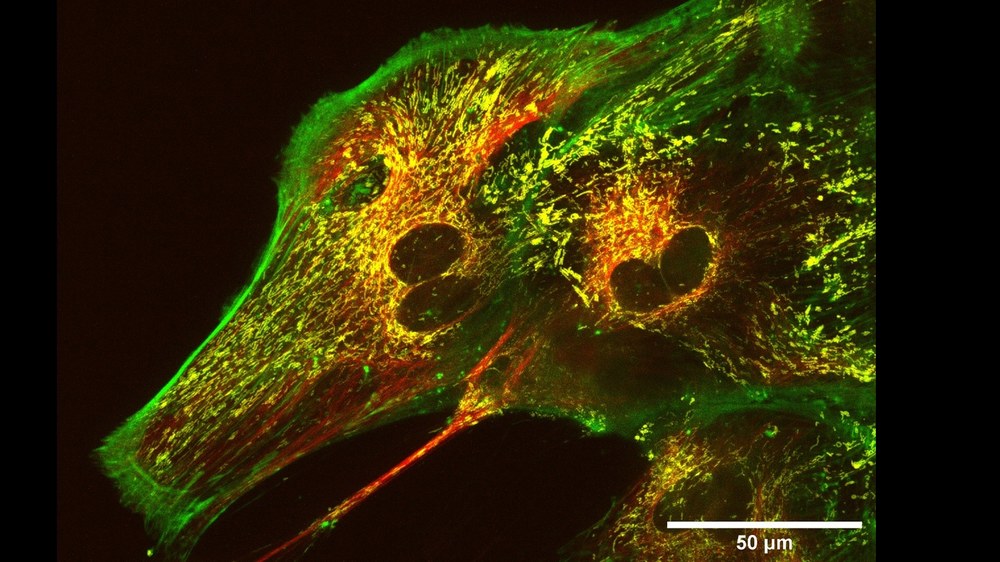
During the LAARA experiment, FLUMIAS will be used on board the ISS from 2026 to investigate parameters of astrocyte reactivity. Astrocytes are the predominant type of glial cells in the brain. These cells have two main functions: in healthy tissue, they support neurons by supplying nutrients and clearing the extracellular space of excess neurotransmitters and waste products. In the event of injury to the brain or nervous system, astrocytes in the surrounding tissue change their phenotype and become reactive, proliferating and migrating to the site of injury, where they form the glial scar through reactive astrogliosis. This process has an inhibitory effect on the regeneration of the injured neural tissue. These processes are highly dynamic and subject the neuronal cells to far-reaching morphological and therefore cytoskeletal adaptations. In previous studies, we have found that these changes in the cells, e.g., how quickly and how many astrocytes become reactive, depend on the gravitational environment. The LAARA experiment now aims to find out whether weightlessness could have negative effects on astronaut brain health.
The factors examined microscopically with FLUMIAS include morphological characteristics, migration behaviour and the dynamics of intracellular actin fibers, the microtubule network and mitochondrial transport. Astrocytes adapted to microgravity will be examined on the FLUMIAS microscope during re-adaptation to different gravitational conditions and during a second adaptation phase back to microgravity in order to investigate adaptive changes in the cytoskeleton. In addition, all parameters are examined in ground-based verification studies using the FLUMIAS Science Reference Model and special incubator centrifuges. The results could reveal potential influences on the cognition and motor performance of astronauts and lead to therapeutic interventions for patients on Earth as well.
Gravity-induced changes in neural transmission using human neuromuscular organoids
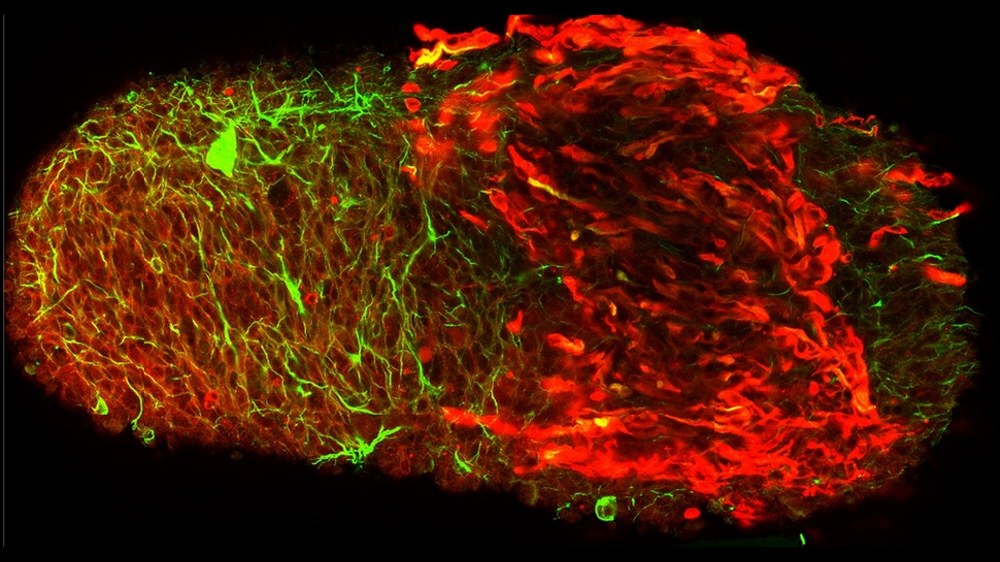
Weightlessness in space can lead to far-reaching physiological changes in the human body, in particular muscle atrophy and loss of bone mass. Despite intensive physical activity, astronauts can lose up to 20% of their calf muscle mass within just two weeks. A key factor that is not yet fully understood is the role of neuromuscular junctions (NMJs), which mediate communication between motor neurons and muscle cells. Changes in gravity affect the viscosity of neuronal membranes, among other things, and can thus impair neuronal signal transmission and synaptic function. Direct investigation of human NMJs in space has not been possible until now due to limited access, which is why research relies heavily on animal models. An innovative and forward-looking approach is therefore the use of human neuromuscular organoids (NMOs) generated from induced pluripotent stem cells (iPSCs). These enable the investigation of the effects of altered gravitational conditions on human NMJs under controlled laboratory conditions. The aim is to further optimise the development of these complex organoid systems in order to generate complex tissue structures and better understand the mechanisms of muscle degenerative diseases that are influenced by altered neural signal transmission.
Our research focuses on understanding the underlying molecular mechanisms of cellular gravity perception in iPSC-derived cell types, particularly those involved in neuromuscular connection and in cerebral and retinal organoids. A central, as yet unanswered question that arose during the investigation of muscle deconditioning is the influence of altered neural signal transmission as the basis for reduced muscle activity.
To establish a robust model system, NMOs are cultivated in 3D Petri dishes, which enable the independent formation of spheroidal 3D microtissues. These neuromuscular organoids replicate functional miniature models of the connection between nerve and muscle cells and are ideal for researching neuromuscular diseases and developing new therapeutic approaches.
In addition to investigating molecular mechanisms of gravity perception in cells and human subjects, another focus is on the individual reprogramming and cultivation of human induced pluripotent stem cells (iPSCs). The long-term goal is to perform multi-omics analyses of personalised cell types from various space studies.
Related Links
Contact
Dr. rer. nat. Christian Liemersdorf
Dr. rer. nat. Patrick Lau
