Sulphurreal

iStock.com/SIAATH
A thermochemical cycle based on sulphur for long-term storage of solar energy
Duration: 1.10.2023 - 30.9.2026
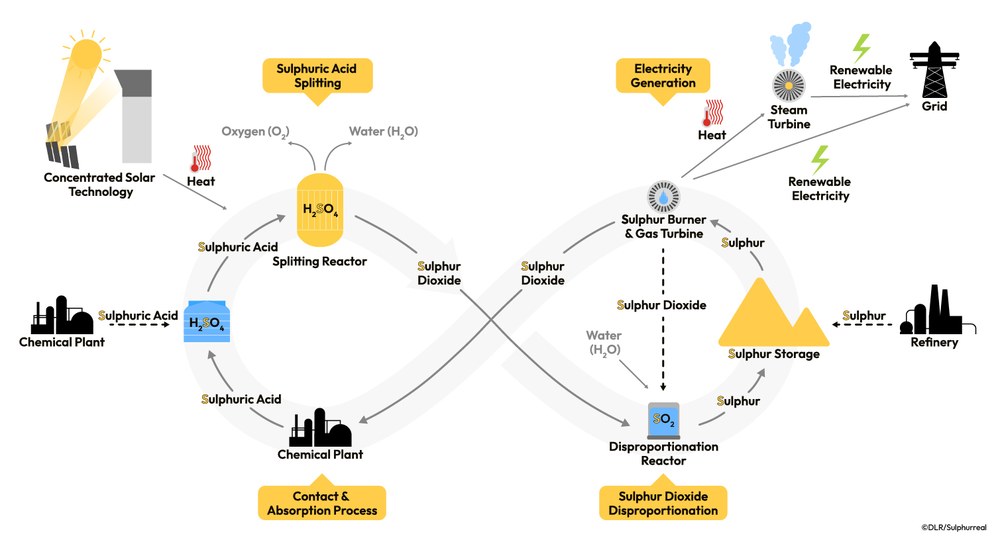
The Sulphurreal project presents an innovative solution for directly storing solar energy in solid elemental sulphur. This is achieved by using concentrated solar radiation to provide the thermal energy for the conversion of sulphuric acid into sulphur. The sulphur can then be used on demand as a fuel, releasing the stored solar energy as heat to drive a gas turbine and produce electricity.
The Sulphurreal cycle is built on a three-step process that harnesses and stores solar energy, providing a reliable and sustainable power source. In the first step, renewable heat energy vaporizes sulphuric acid (H2SO4) in a chemical reactor at temperatures between 650-900 degrees Celsius and splits it into sulphur dioxide (SO2), oxygen, and steam. In regions with sufficient high direct solar irradiation, concentrated solar heat from a solar thermal plant could be used as energy source.
Next, the sulphur dioxide reacts with water in a disproportionation reaction. This produces elemental sulphur and sulphuric acid, which can be recycled back into the first step.
Hence, a significant portion of the solar energy used to drive the sulphuric acid splitting can be stored in the form of Sulphur. This offers several advantages: Sulphur can serve as energy storage medium over long periods of time and can be easily transported.
In the third step of the cycle, Sulphur is combusted when and where needed to release the energy in the form of high-temperature heat of more than 1,200 degrees Celsius, suitable for gas turbines and allowing highly efficient combined cycle power generation (gas turbine and steam turbine).
Closed Sulphurreal-Cycle is efficient and sustainable
The combustion product sulphur dioxide is fed back into the cycle, where it can either be converted back into sulphuric acid in an already known contact and absorption process or reused for sulphur production in the disproportionation step.
In the first case, investigated in this project, the process operates in a closed cycle and regenerates sulphuric acid, making it efficient and sustainable. Alternatively, in the second case the cycle can operate as an "open" one, sourcing further sulphuric acid and sulphur from large-scale industrial processes as the feedstock (dotted arrows in the schematic). In either case all intermediate gas products like sulphur dioxide are recirculated without releasing them into the atmosphere.
Integration of renewable energies into industrial processes
Sulphur, the ninth most abundant element on Earth, is a significant by-product of natural gas desulphurisation in the oil industry. Approximately 70 million tonnes of sulphur are produced annually, mostly as a by-product of crude oil and natural gas processing. Sulphuric acid, on the other hand, is the world’s most widely produced chemical. It serves as a vital raw material for numerous industries, including fertilisers, explosives, dyes, plastics, and pharmaceuticals.
The results of the Sulphurreal project can offer decisive advantages to the industry in terms of energy efficiency and sustainable utilisation of raw materials. The direct storage of solar energy in solid elemental sulphur not only reduces dependence on fossil fuels, but also promotes the integration of renewable energies into existing industrial processes. The knowledge gained can create the basis for future developments that are economically viable and ecologically compatible and contribute in diversifying our society’s future portfolio of energy mix.
More information on the projectwebsite:

EIC
Project | Sulphurreal |
|---|---|
Duration | 1.10.2023 - 30.9.2026 |
Project participants |
|
Funding |